What we do
Sroware helps startups and businesses of all sizes build scalable, high-performing digital products. From sleek marketing websites to full-stack SaaS platforms, we turn your ideas into powerful, user-friendly software—on time and on budget.


Mobile App Development
Cross-platform mobile apps for iOS and Android with seamless UX, API integrations, and App Store deployment
SaaS Platforms
We specialise in full-stack SaaS—from MVP to global rollout. Cloud-hosted, secure, API-first platforms built to perform.
Web Applications
Robust web apps built using modern frameworks like React, Angular, and .NET—designed to scale with your business.
Our process
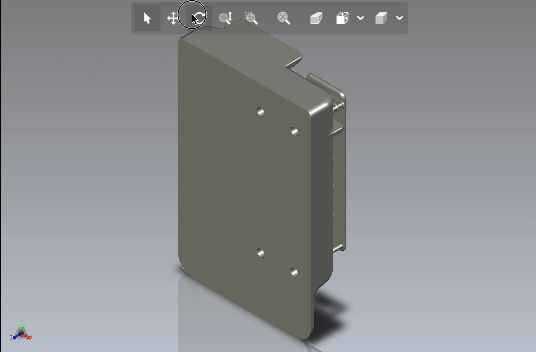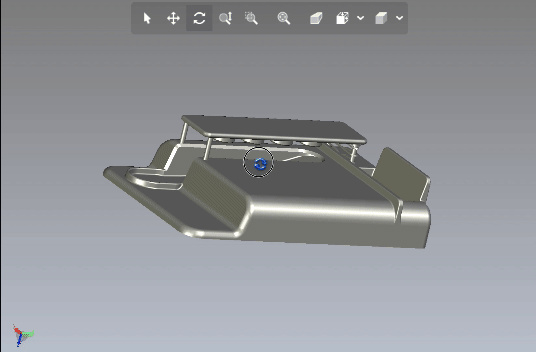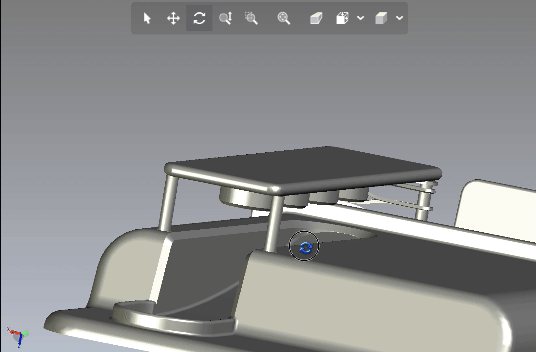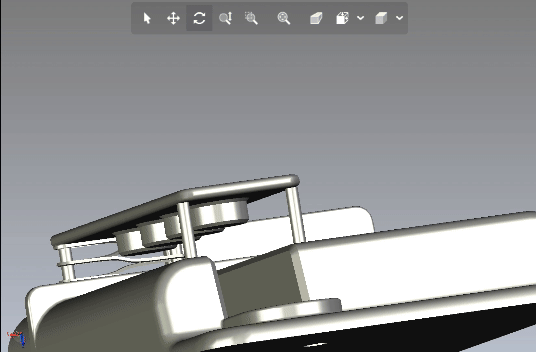
Creating scalable software platforms and innovative hardware devices for startups.
Concept to Commercialisation
Developing cutting-edge solutions for any industry.

Sroware is our go-to technical partner for everything from bespoke software to platform certification.
Better Day Health

The team at Sroware exceeded our expectations in creating a scalable software solution for our medical technology startup.
QuickTake Health
★★★★★
★★★★★
Innovate with us.
Development of scalable software platforms and medical devices.
DUBLIN
KAUNAS
Dun Laoghaire
Co. Dublin, Ireland
Glasshouse GH2
© 2025. All rights reserved.
LT-51423
Kaunas, Lithuania
K. Barsauskas st. 59,